BCAAs & THE SCIENCE

A name recognized across the entire fitness industry: The branched chain amino acids, or, BCAA's. This supplement is known to have a key role in protein synthesis. Pre-workout, intra-workout, post-workout, with your coffee, middle of the night—you can find all kinds of creative ways to incorporate BCAAs into your supplement stack. But what are BCAAs? What is muscle protein synthesis? How does it work and what role do BCAAs have in it? To what degree? There's some fundamental questions that we're going need answered if we're going to be adding this supplement to our shopping cart frequently. So let's take a look at what they are, how they function, their use as a supplement, and the proper dosing if you do choose to supplement them.
WHAT IS IT?
BCAAs are comprised of the three essential amino acids: Leucine, Isoleucine, and Valine (1,2,3). They're all pictured below (Spoiler alert: the only important one is Leucine).
I'm kind of kidding, but not really—I'll expand on that in a minute. Most BCAA supplements will say "supports muscle recovery and growth" or something along those lines. Their justification for this true claim is the literature that has shown that intake of BCAAs:
- Increases muscle protein synthesis signaling (MPS) (4,5,6,7,8,9,10,11)
- Decreases muscle protein breakdown (MPB) (7,9,11)
What do these terms mean? How does this happen? And how significant of an increase/decrease? Let's define a few terms first. Muscle protein synthesis (MPS) is the creation of new proteins for muscle tissue. There is a very complicated chain of phosphorylation reactions that up-regulate this nutrient sensitive process. Muscle Protein Breakdown (MPB) is the opposite. This is the breaking down of muscle protein to create pools of free amino acids. Both are important for muscle remodeling, adaptation to training, and regulation of muscle mass (7).
Next question that should be in your head: How? What's the mechanism? Meet mTOR. mTOR is the mechanistic (or mammalian) Target of Rapamycin. Commonly cited in literature as mTORC-1 (mechanistic target of rapamycin complex-1 because there are 2 of them). Complex in this instance just means a large group of protein kinases. A protein kinase is an enzyme that facilitates a phosphorylation reaction. So we have two big bundles of proteins that facilitate reactions that result in a downstream signaling of MPS so we can get big and strong (7, 8, 12). Are you a visual learner? Here's a picture of the process we're discussing: 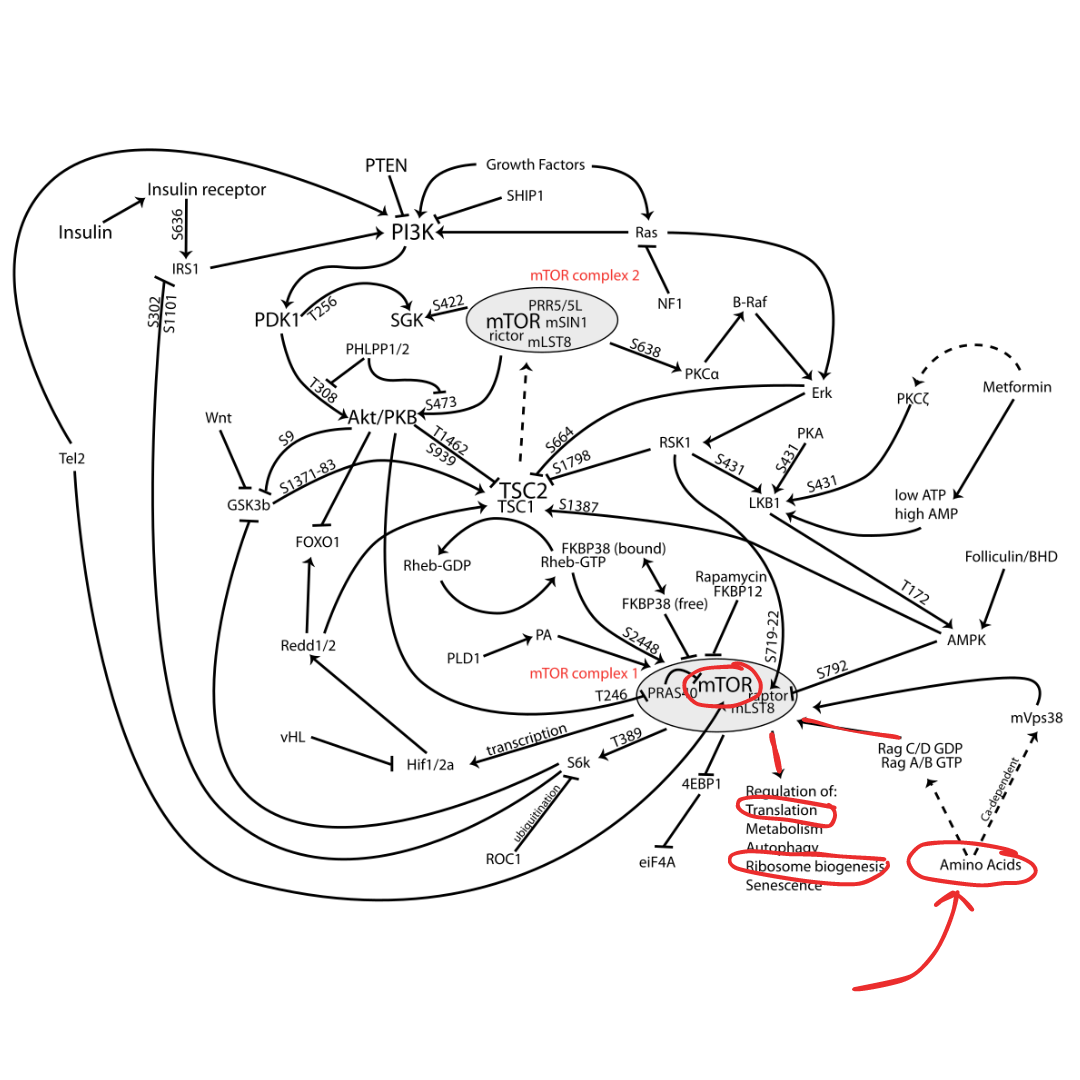 Figure 2. The mTOR pathway (I outlined the items that are relevant for our discussion in red. We'll save the rest for another time)
Figure 2. The mTOR pathway (I outlined the items that are relevant for our discussion in red. We'll save the rest for another time)
So what do the BCAAs have to do with mTORC-1? Glad you asked. Leucine directly stimulates mTOR. Pretty awesome. Leucine can directly and independently stimulate mTOR. mTOR gets activated, so does muscle protein synthesis signaling. So, Leucine directly and independently up-regulates muscle protein synthesis signaling (3,12,13). What about Isoleucine and valine? Well, they're just along for the ride.
Okay, so, leucine directly activates mTOR which activates MPS signaling, what about MPB (muscle protein breakdown)? Well, literature has shown that Leucine can stimulate an insulin response at certain doses. The current hypothesis is that the presence of insulin inhibits muscle protein breakdown due to its anabolic nature (anabolic = build things up). So it's not that leucine acts directly on any enzyme to inhibit MPB, it just recruits some help from some hormones. Leucine doesn't stimulate the release of insulin like glucose, but we'll get there in a second.
Leucine —> +mTOR —> +MPS signaling = grounds for protein anabolism (~HYPERTROPHY~)
THE APPLICATION
Alright, enough background on convoluted molecular biological pathways in the body. Should we buy and take BCAAs or not? For those here just to know the answer, no. For those who have a burning curiosity to understand like me, let's continue.
To make sure we haven't left any soldiers behind, let's recap: Branched chain amino acids are comprised of three essential amino acids—Leucine, Isoleucine, and Valine. Leucine directly and independently stimulates mTORC-1 which activates muscle protein synthesis signaling which is the biological process of building new muscle proteins to help repair and grow. So the claims on the side of the label are true! Why am I saying we shouldn't take BCAAs?
Guess what else stimulates and activates muscle protein synthesis via the same process and pathways? Eating food. Classic. Remember how in the beginning I alluded to the fact that research is often taken out of context and applied to whatever context people want to apply it in? This is a great example. Yes all of the claims and supporting literature is true, but now we should be asking ourselves what question? Exactly—where's the application? Hint: it's not in BCAA supplementation.
Alright, I'm being a bit harsh on the supplement industry. Leucine/BCAA supplementation does indeed have it's place. You know whey protein? Yeah, that stuff has BCAAs in it. Now, leucine is arguably the most important amino acid for turning on muscle protein synthesis and that's why any good supplement will have a 2:1:1 ratio of Leucine to Isoleucine and valine. This supports the leucine trigger hypothesis which basically states that incorporating a higher dose of leucine in tandem with a complete amino acid mixture, meaning all 20 amino acids, will provide the perfect canvas for maximizing our protein anabolism (6, 14, 15).
**Protein anabolism: the actual building of proteins.
You'll notice earlier in the paper I kept referring to muscle protein synthesis signaling. That's because the signaling of the process and the process playing out are very different. Leucine directly stimulates the signaling of MPS which is cool, but in order for there to be anabolism of protein, we need a free pool of amino acids to pull from. We need an adequate amount for net positive protein balance. Then we can have growth. If we don't have an adequate amount, we will have net negative protein balance.
Only 0.5 to 1.0% of the total amino acid content of the body is present as free amino acids, in plasma, or the intracellular and extracellular spaces [12]. However, the small amounts of amino acids present in the ‘free pools’ are responsible for the metabolic or substrate influences of all amino acids (9). Where do we get these amino acids that make up the free pools? Well, the 11 non-essential ones we can make, other 9 essential amino acids can come from two places:
- Food (exogenous - outside)
- Muscle protein (endogenous - from the inside)
Muscle protein is the only endogenous (internal) source of essential amino acids. So, if we're in a non-fed state and consume leucine alone, we would activate muscle protein synthesis signaling. This signal would tell us we need amino acids to build up muscle protein but without an adequate source, would breakdown muscle protein to get the essential amino acids a.k.a net negative protein balance (14).
Let's picture the process like baking a frozen pizza. You set the oven to bake at 400°F—that's the signaling. The machinery is now ready to perform the task (bake our pizza). You set a timer for 20 minutes, come back and open the oven to see there's no pizza. Turns out you didn't give the oven a pizza to bake... You just wasted electricity for the oven to bake itself. Leucine (or BCAAs (but really just leucine)) signals to turn on the muscle protein oven (mTOR). If you don't provide mTOR with the pizza (an adequate pool of free amino acids), it just sits there and bakes itself (breaks down muscle protein to create a pool of free amino acids to use to build muscle). Then you wonder why you aren't JACKED.
How do you avoid this? Simple: sources of complete or essential amino acids. This provides us with a ready to go pool of amino acids for protein anabolism. There is some research that suggests slightly higher doses of leucine than contained in most protein powders can help further stimulate muscle protein synthesis and over a longer period of time (2, 3, 6, 9, 12).
TO BRING IT ALL HOME
Leucine is the only important amino acid in the BCAAs when it comes to increasing MPS signaling. Leucine turns the MPS machinery on and the MPB machinery off. It also increases sensitivity and subsequently the efficacy of insulin in anabolism. This in tandem with a complete mixture of amino acids and glucose provides the perfect canvas for optimal muscle protein synthesis. You need a complete source of amino acids for full saturation of muscle protein anabolism. If you supplement with less than 9 of the essential amino acids via taking a BCAA supplement on it's own, the literature tells us you will likely create net negative protein balance which means muscle protein breakdown.
So buy some whey isolate and some leucine. After your workout toss about 2.5g of Leucine in with your whey, eat a banana, and you'll be so anabolic it's crazy. Happy supplementing.
REFERENCES
- Neinast M, Murashige D, Arany Z. Branched Chain Amino Acids. Annu Rev Physiol. 2019 Feb 10;81:139-164. doi: 10.1146/annurev-physiol-020518-114455. Epub 2018 Nov 28. PMID: 30485760; PMCID: PMC6536377.
- Shad, B. J., Thompson, J. L., & Breen, L. (2016). Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. American Journal of Physiology-Endocrinology and Metabolism, 311(5), E803–E817. doi:10.1152/ajpendo.00213.2016
- Mero, A. (1999). Leucine Supplementation and Intensive Training. Sports Medicine, 27(6), 347–358. doi:10.2165/00007256-199927060-00001
- Chan, A. H., D’Souza, R. F., Beals, J. W., Zeng, N., Prodhan, U., Fanning, A. C., … Mitchell, C. J. (2019). The Degree of Aminoacidemia after Dairy Protein Ingestion Does Not Modulate the Postexercise Anabolic Response in Young Men: A Randomized Controlled Trial. The Journal of Nutrition. doi:10.1093/jn/nxz099
- Li, J. B., & Jefferson, L. S. (1978). Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochimica et Biophysica Acta (BBA) - General Subjects, 544(2), 351–359. doi:10.1016/0304-4165(78)90103-4
- Churchward-Venne, T. A., Burd, N. A., Mitchell, C. J., West, D. W. D., Philp, A., Marcotte, G. R., … Phillips, S. M. (2012). Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. The Journal of Physiology, 590(11), 2751–2765. doi:10.1113/jphysiol.2012.228833
- Tipton, K. D., Hamilton, D. L., & Gallagher, I. J. (2018). Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans. Sports Medicine, 48(S1), 53–64. doi:10.1007/s40279-017-0845-5
- Saxton, R. A., & Sabatini, D. M. (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell, 168(6), 960–976. doi:10.1016/j.cell.2017.02.004
- Dardevet, D., Sornet, C., Bayle, G., Prugnaud, J., Pouyet, C., & Grizard, J. (2002). Postprandial Stimulation of Muscle Protein Synthesis in Old Rats Can Be Restored by a Leucine-Supplemented Meal. The Journal of Nutrition, 132(1), 95–100. doi:10.1093/jn/132.1.95
- Kimball, S. R., & Jefferson, L. S. (2004). Molecular mechanisms through which amino acids mediate signaling through the mammalian target of rapamycin. Current Opinion in Clinical Nutrition and Metabolic Care, 7(1), 39–44. doi:10.1097/00075197-200401000-00008
- Garlick, P. J. (2005). The Role of Leucine in the Regulation of Protein Metabolism. The Journal of Nutrition, 135(6), 1553S–1556S. doi:10.1093/jn/135.6.1553s
- Anthony, J. C., Yoshizawa, F., Anthony, T. G., Vary, T. C., Jefferson, L. S., & Kimball, S. R. (2000). Leucine Stimulates Translation Initiation in Skeletal Muscle of Postabsorptive Rats via a Rapamycin-Sensitive Pathway. The Journal of Nutrition, 130(10), 2413–2419. doi:10.1093/jn/130.10.2413
- Biolo, G., Tipton, K. D., Klein, S., & Wolfe, R. R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology-Endocrinology and Metabolism, 273(1), E122–E129. doi:10.1152/ajpendo.1997.273.1.e122
- Stokes, T., Hector, A., Morton, R., McGlory, C., & Phillips, S. (2018). Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients, 10(2), 180. doi:10.3390/nu10020180
- Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107(3):987–92.
SUBSCRIBE FOR WRITINGS RIGHT TO YOUR INBOX
I take a lot of time to make sure they're good. I promise.
I hate SPAM. I will never sell your information, for any reason.
